
Anastrepha and Toxotrypana:
descriptions, illustrations, and interactive keys


|
Anastrepha and Toxotrypana:
|

|
Vestiture • Thorax • Wing • Abdomen • Female terminalia • Male terminalia • Egg • Miscellaneous • References
An explanation is provided for each morphological character used in this system. It may be accessed in Intkey by clicking the ‘Notes’ button on the character image, whereas in Lucid it is part of the character ‘help’ page. Help images, with relevant morphological parts labeled, may be accessed in Intkey via the ‘Subject’ menu in the upper left corner of the character image window. In Lucid the help image forms the lower part of the character ‘help’ page.
Additional explanations of the morphological terminology for fruit flies, along with the same labeled help images, are provided in this document. The system follows the terminology of the White et al. (1999) glossary, from which most of the entries below were copied. For additional morphological terms or explanations, see the excellent treatments of general fly morphology by McAlpine (1981) and Cumming & Wood (2009). Users of this system are presumed to be familiar with general insect morphology and basic terms, such as dorsal, ventral, and lateral, that can be found in any general textbook of entomology. The entries include some common but outdated or less preferred morphological terms that might be encountered in the broader tephritid literature.

Primary parts of body
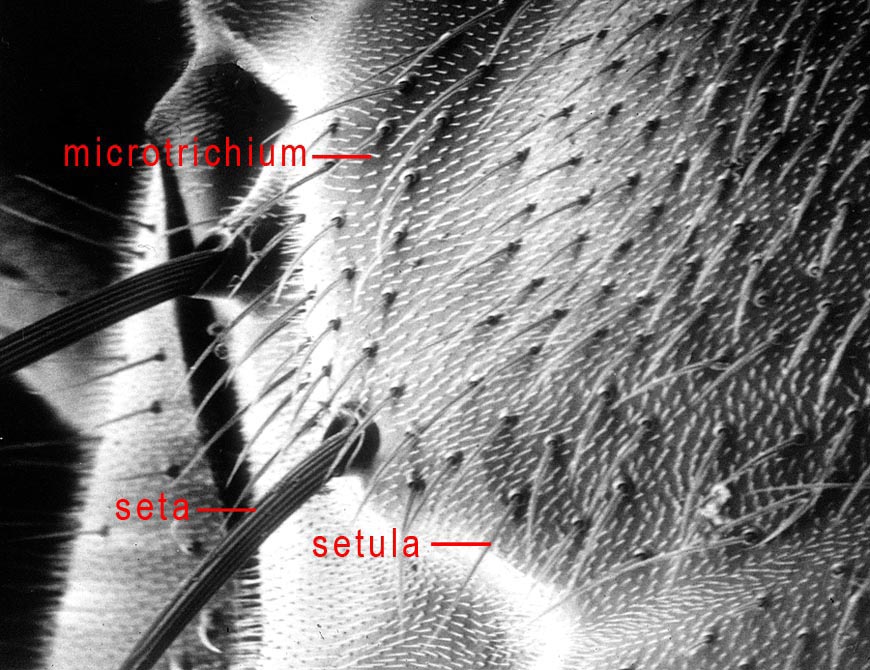
Vestiture
BRISTLE – An alternative term for a large macrotrichium, or seta.
CONVERGENT SETA – A seta that is inclinate, i.e., leans towards the midline of the fly. The Terelliini, for example, have the posterior pair of orbital setae convergent.
MICROTRICHIA/MICROTRICHOSE – Usually minute projections of the cuticle that lack alveoli. Microtrichia are usually hair-like or scale-like under compound or scanning electron microscopy. They may cover parts of the wing membrane, making them look darker, or parts of the body. On the body, depending upon their shape and density, they may produce a subshining, silvery or other colored, or dull, matt appearance. Many species have patterns, especially on the scutum, of bare and microtrichose areas, or due to variation in microtrichia density or shape. The appearance of these patterns may change depending on the angle of view, and they are usually not visible in specimens in fluid or may be obscured in specimens dried from fluids. The terms pollinose, pruinose, and microtomentose have also been used to describe microtrichose areas. Also see seta. The scutal microtrichia should not be confused with the scutal setulae.
POLLINOSITY /POLLINOSE – See microtrichia.
PROCLINATE SETA – Any seta which lean forwards.
PRUINESCENCE / PRUINOSITY / PRUINOSE – See microtrichia. The term pruinose was used by McAlpine (1981) to describe body surfaces covered by microtrichia, but as noted by Sabrosky (1983), pruinose means covered with a white, powdery substance and is no more appropriate than other terms such as pollinose.
RECLINATE SETA – Any seta which leans backward.
SENSILLUM (plural: SENSILLA) – Simple sense organs, e.g., on the larva or on the aculeus tip.
SETA/SETULA (plural: SETAE/SETULAE) – Hairlike surface structures that are articulated (i.e., having alveoli, or sockets) are macrotrichia. A relatively large one is called a seta (e.g., the acrostichal and dorsocentral setae on the scutum), and a smaller one is called a setula (e.g., the scutal setulae that cover much of the scutum). The setulae should not be confused with the much smaller microtrichia that are often present on the scutum and other parts of the body.
TOMENTOSE/TOMENTOSITY/TOMENTUM – See microtrichia.
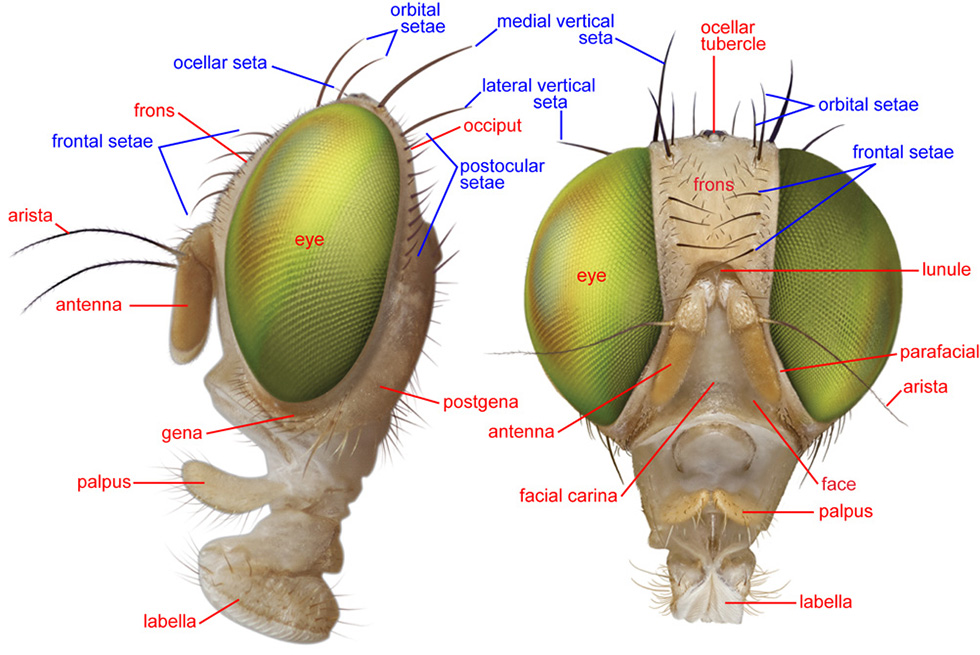
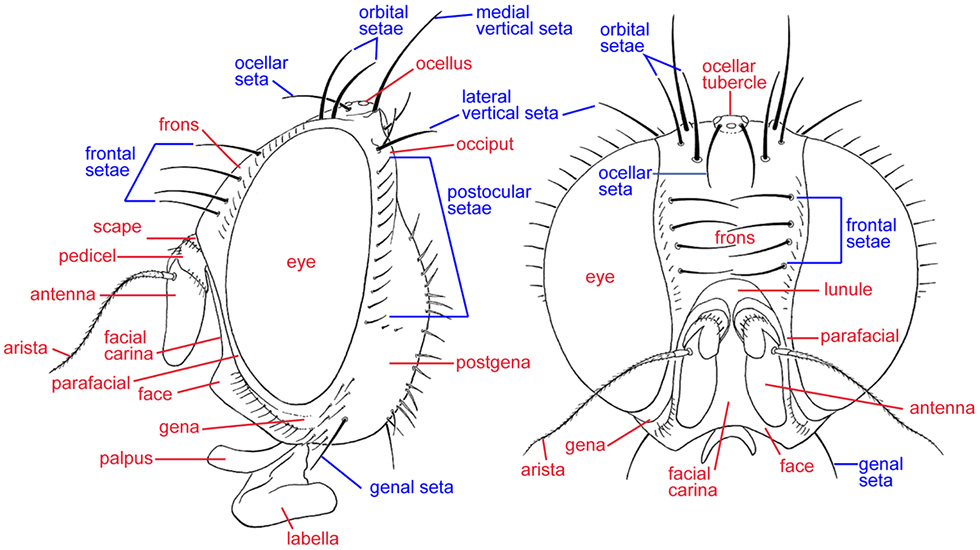
Head, lateral and anterior
ARISTA – In Tephritidae, the flagellum is modified into a large first flagellomere and 3 very slender ones that arise dorsally near the base of the first, and form the style- or seta-like arista. In most tephritids the arista is pubescent, i.e., with minute hairlike projections.
CLYPEAL RIDGE – See facial carina.
FACIAL CARINA – A keel-like medial protrusion of the face. It has erroneously been called the clypeal ridge (e.g., Stone 1942).
FIRST FLAGELLOMERE – In Tephritidae, the apparent third segment of the antenna (see arista). It has also been called the postpedicel. It is occasionally elongate (e.g., some species of Anastrepha daciformis group).
FRONS – The anterodorsal area of the head, bounded laterally by the eyes, posteriorly by the vertex, and anteriorly by the antennae.
FRONTAL SETAE – The row of setae next to each eye on the lower part of the frons. In Tephritidae they are usually all incurved, and most species have between 2 and 5 pairs, i.e., a row of 2-5 setae next to each eye. They have also been called inferior or lower fronto-orbital setae.
GENA (plural: GENAE) – The area ventral to the eye, posterior to the parafacial and facial ridge, and anterior to the postgena.
GENAL SETA – In general, any setae on the gena. In Tephritidae there is usually one seta larger than the surrounding setulae that is called the genal seta.
INNER VERTICAL SETA – See vertical setae.
INFERIOR FRONTO-ORBITAL SETAE – See frontal setae.
LATERAL VERTICAL SETA – See vertical setae.
LOWER FRONTO-ORBITAL SETAE – See frontal setae.
LUNULE – The semicircular plate above the antennal bases and below the ptilinal fissure.
MEDIAL VERTICAL SETA – See vertical setae.
OCELLAR SETA – A paired seta in front of the posterior ocellus. It is small and weak in Toxotrypana and most species of Anastrepha.
OCELLAR TUBERCLE – The raised, rounded area that encloses the three ocelli, which are arranged as a triangle.
ORBITAL SETAE – Setae on the upper, lateral part of the frons. In Tephritidae there are usually two, although the posterior pair are sometimes absent. They are reclinate in Anastrepha and Toxotrypana. These setae have also been called superior or upper fronto-orbital setae.
OUTER VERTICAL SETA – See vertical setae.
PEDICEL – The second segment of the antenna.
POSTOCELLAR SETAE – 1-2 pairs of setae behind the ocellar tubercle.
POSTOCULAR SETAE – The row of small setae posterior to each eye.
PTILINAL FISSURE – The inverted U-shaped slit which runs above the antennal bases, ending in the genal grooves. It marks the edge of the sclerite including the face and lunule that is pushed forwards when the ptilinum is expanded.
PTILINUM – A sack-like structure which is inflated by the adult as a mechanism for bursting the puparium. It folds back inside the head soon after the fly emerges.
SUPERIOR FRONTO-ORBITAL SETAE – See orbital setae.
UPPER FRONTO-ORBITAL SETAE – See orbital setae.
VERTEX – The uppermost part of the head, between the eyes and around the ocellar triangle.
VERTICAL SETAE – There are two pairs of vertical setae on or slightly posterior to the dorsalmost part of the head, or vertex, near the margin of the eye. The medial vertical seta is reclinate and/or inclinate. The lateral vertical seta is usually lateroclinate. McAlpine (1981) termed these setae the inner vertical seta and outer vertical seta, respectively, but as they are external in both cases the adjectives medial and lateral are more appropriate.
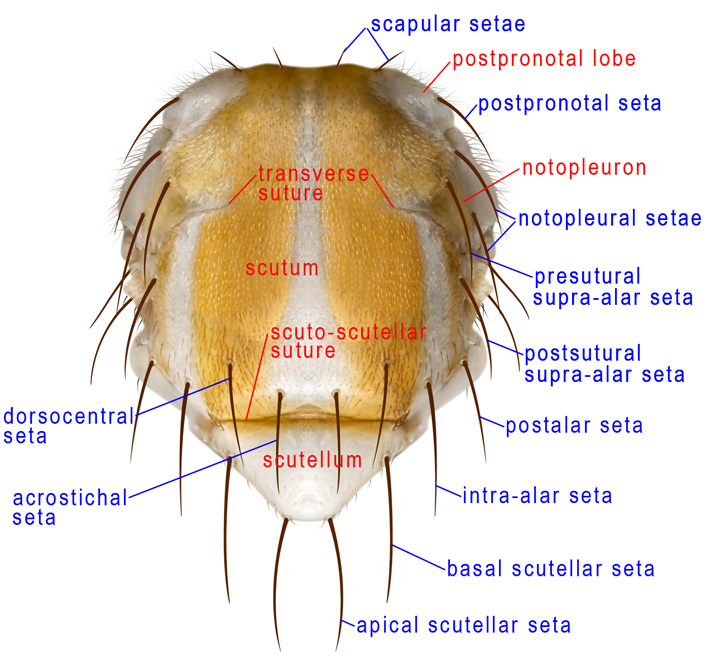
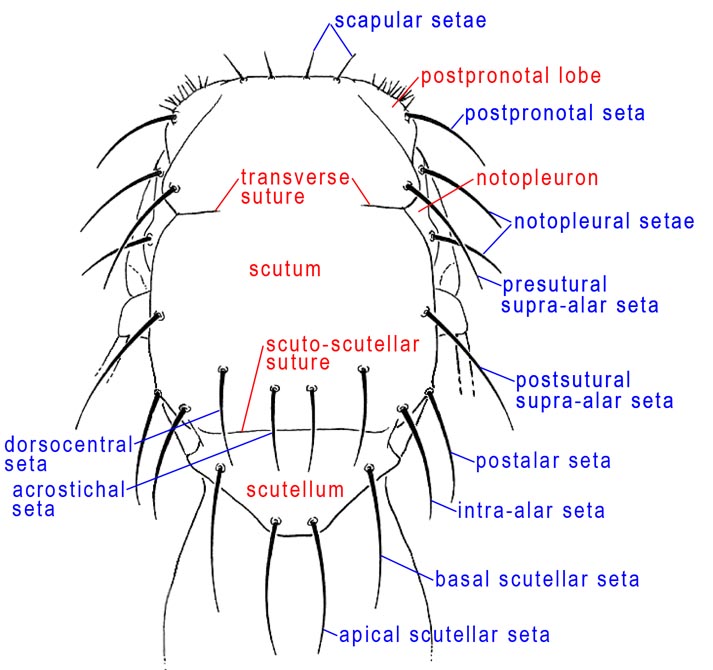
Thorax, dorsal
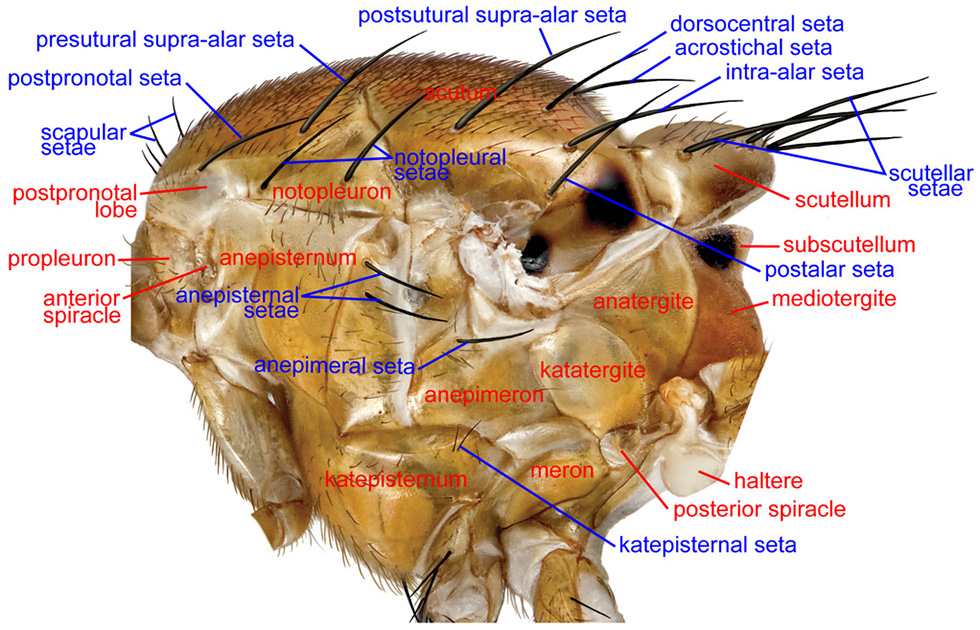
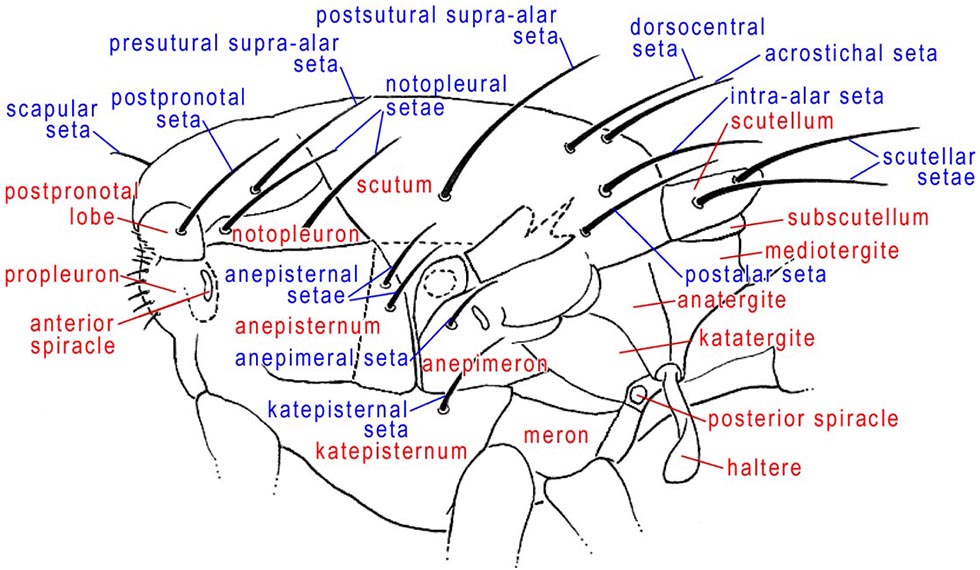
Thorax, lateral
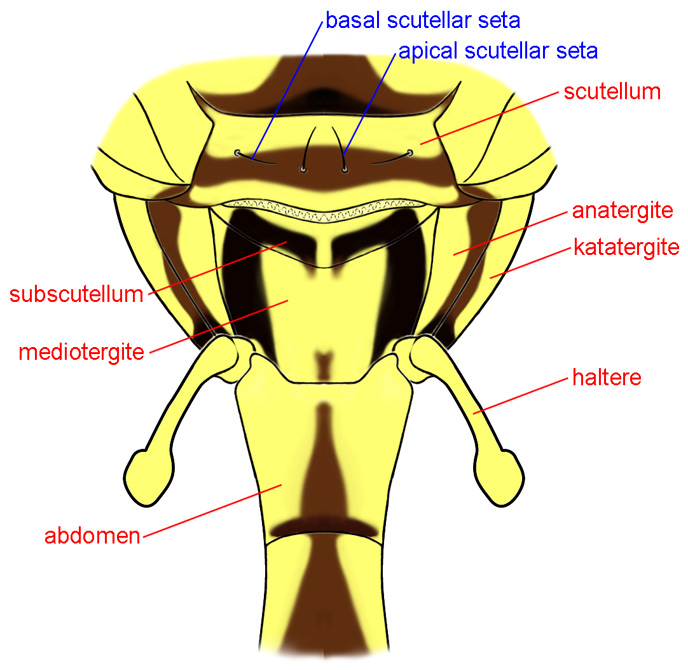
Thorax, posterior
ACROSTICHAL SETA – In Diptera, the row or rows of setae nearest to the midline of the scutum. Tephritidae have at most one pair of these setae, placed just anterior to the scuto-scutellar suture. Consequently, some authors have called them the prescutellar setae. They are rarely absent in Anastrepha but are minute or absent in Toxotrypana.
ANATERGITE – In lateral view, this sclerite is anterior to the mediotergite and above the haltere. The anatergite and katatergite together form the laterotergite, which was termed the pleurotergite or hypopleural callus by many authors.
ANEPIMERON – The lateral thoracic sclerite that is below the wing base. It has also been called the pteropleuron. In Tephritidae it bears an anepimeral seta.
ANEPISTERNAL PHRAGMA – In Tephritidoidea the anepisternum has a vertical phragma just anterior to the series of setae near the posterior edge. This phragma, which has also been called the mesopleural suture, is usually visible in the Phytalmiinae and Trypetinae, but is obscured by dense microtrichia in most Tephritinae (in the latter it can usually be seen if the anepisternum is wetted with a drop of alcohol).
ANEPISTERNUM – The large pleural sclerite of the thorax between the anterior spiracle and the wing base. It has also been called the mesopleuron. In Tephritidae, near the posterior margin, it bears 1 or a row of anepisternal setae or setulae which decrease in size ventrally.
DORSOCENTRAL SETAE – In Diptera, a series of paired setae between the acrostichal and intra-alar series. Tephritids have at most two pairs, and their number and position are useful in higher classification. There is usually one postsutural dorsocentral seta in Anastrepha more or less aligned with the postsutural supra-alar seta. It is weak or absent in Toxotrypana. The dorsocentral seta should not be confused with the acrostichal seta, which is closer to the midline of the scutum.
GREATER AMPULLA – A small, dome-shaped area on the anterodorsal part of the anepimeron in front of the wing base. It is variably produced, but present, in most Tephritidae.
HUMERAL CALLUS or LOBE – See postpronotal lobe.
HUMERAL SETA – See postpronotal seta.
INTRA-ALAR SETAE – A series of setae between the dorsocentral and supra-alar series; tephritids have only one pair of intra-alars, placed near the level of the acrostichal setae.
KATATERGITE – The lateral thoracic sclerite anteroventral to the anatergite and between the wing base and the posterior spiracle. In Tephritidae it is more produced than surrounding sclerites. Also see anatergite.
KATEPISTERNUM – The triangular sclerite between the coxae of the fore- and midlegs. Most tephritid species have a well developed katepisternal seta near the posterodorsal corner, but it is often weak or absent in Anastrepha and Toxotrypana. This sclerite has also been called the sternopleuron.
MEDIOTERGITE – The sclerite below the scutellum and subscutellum. Some authors have called this the metanotum (e.g., Stone 1942), mesophragma, or the postnotum, but it is only a part of the latter.
MESONOTUM – In flies most of the thorax is derived from the mesothorax, and the mesonotum, which includes the scutum, scutellum and postnotum, forms most of the dorsum. In Tephritidae, the postpronotal lobes are the only dorsally visible parts of the thorax that are not part of the mesonotum. The length of the mesonotum in dorsal view is that of the scutum + scutellum.
MESOPLEURON – See anepisternum.
METANOTUM – See mediotergite.
NOTOPLEURON – A lateral sclerite on the mesonotum, derived from the scutum. In Tephritidae, it bears two setae, the anterior and posterior notopleural setae. The color of the notopleuron is sometimes useful as a character in darker bodied Anastrepha) species.
PLEURON (plural: PLEURA) – The lateral side of a body part or segment, as in the thoracic pleuron.
PLEUROTERGITE – See anatergite.
POSTALAR SETA – The posterolateralmost seta on the scutum (Foote et al. 1993). In some Diptera one or more postalar setae occur on a distinct postalar callus, but that callus is not differentiated in Tephritidae and so the homology of this seta is uncertain. Some authors have interpreted it as a posterior supra-alar (e.g., Drew 1989, White & Elson-Harris 1992), but it is more lateral than the supra-alar line (this is more obvious in lateral view) and in the typical position, on the posterolateral corner of the scutum, of a postalar seta.
POSTPRONOTAL LOBE – The anterolateral ‘shoulder’ of the thorax known in earlier works as the humeral lobe or humeral callus. In most Tephritidae it bears one postpronotal seta, which has also been called the humeral seta.
POSTSUTURAL VITTAE – See scutal vittae.
PRESCUTELLAR SETA – See acrostichal seta.
PRESUTURAL SETAE – See supra-alar setae.
PTEROPLEURON – An old term for the anepimeron.
SCAPULAR SETAE – Small setae, only slightly larger than the scutal setulae, near the anterior margin of the scutum (Munro 1947). There are often 1–2 pairs in Tephritidae.
SCUTAL VITTAE – Diverse scutal color patterns occur across the Tephritidae, and dark vittae (stripes) are not uncommon. Anastrepha species have pale or sometimes bright yellow or white stripes or vittae whose color appears to be determined by internal tissues and often changes with death (they are more or less clear areas in the cuticle termed ‘xanthines’ by Munro). They are usually distinct in dark bodied species, but are often poorly differentiated in pale species, particularly in dried specimens. Most species appear to have a paired sublateral postsutural vitta extending from the transverse suture to the posterior margin on the intra-alar line. Many species have an unpaired medial vitta, and some have presutural lateral and/or dorsocentral vittae. In species of Toxotrypana the scutum is predominantly yellow with dark brown vittae, or predominantly brown. Also see vitta.
SCUTELLUM – The triangular or semicircular sclerite posterior to the scutum. In Tephritidae it bears 1-2, or rarely more, large marginal setae and may have additional setulae. The basalmost marginal seta is called the basal scutellar seta or anterior scutellar or lateral scutellar seta, and the apicalmost is termed the apical scutellar seta or posterior scutellar seta.
SCUTUM – In Tephritidae, most of the thorax visible in dorsal view is the scutum. Only the postpronotal lobe, the scutellum, and the postnotum are not part of it. It includes pre- and postsutural areas incompletely divided by the transverse suture.
STERNOPLEURON – See katepisternum.
SUBSCUTELLUM – The small sclerite, best seen in posterior view of the thorax, below the scutellum and above the mediotergite. It has also been called the postscutellum.
SUPRA-ALAR SETAE – Tephritids have up to three pairs of supra-alar setae in a longitudinal row between the intra-alar and postalar setae. The presutural supra-alar seta (presut spal s), often called simply the presutural seta, is occasionally absent (e.g., in most Toxotrypana). In Anastrepha and Toxotrypana there is one postsutural supra-alar seta. The postalar seta has been regarded as a posterior supra-alar seta by some authors.
TRANSVERSE SUTURE – Calyptrate Diptera have a complete suture across the scutum between the notopleura. In the Acalyptratae, including the Tephritidae, the central part of this suture is absent but the lateral parts are distinct and extend mesally from each notopleuron. This partial suture divides the presutural and postsutural parts of the scutum. It has also been called the notopleural suture.

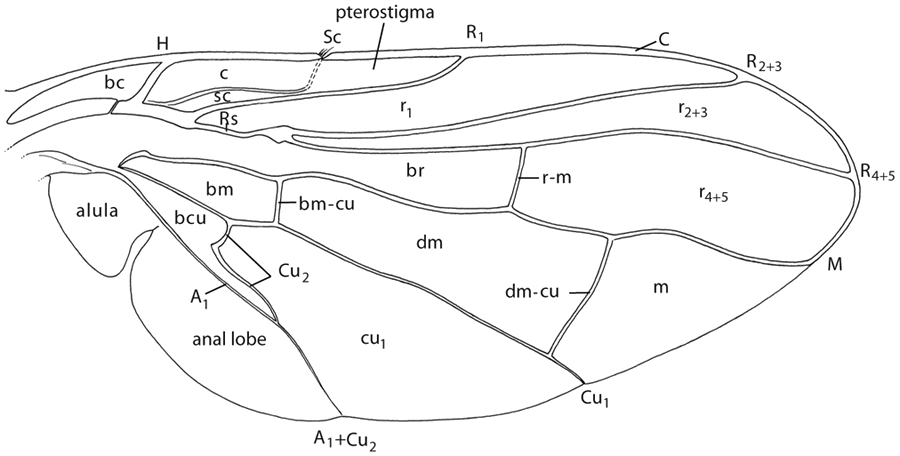
Wing venation

Wing pattern
ANAL CELLS/ ANAL LOBE – The cells posterior to veins A1 and A2 are the anal cells, but they are not closed in Tephritidae and the term anal lobe is more often used for the entire area posterior to vein A1. The term "anal cell" was often incorrectly used in the past for the basal cubital cell.
ANAL CELL EXTENSION – See basal cubital cell.
ANAL STREAK – A diagonal marking that covers cell bcu and part of cell cu1 in most species with a wasp mimicry pattern (e.g., most Dacini, Toxotrypana). It is sometimes called the anal stripe.
ANASTREPHA-TYPE PATTERN – A wing pattern with a short costal band ending at the apex of vein R1, a strongly oblique radial-medial band and an anterior apical band joined to form an S-band, and subapical and posterior apical bands often joined to form a V-band (Lima 1934, Stone 1942).
BASAL CELLS – A general term for the basal radial, basal medial and basal cubital cells (br, bm and bcu). The old terms 1st and 2nd basal cells meant cells br and bm, respectively.
BASAL CUBITAL CELL (cell bcu) – The basal wing cell bounded anteriorly by the base of vein Cu, apically by vein Cu2, and posteriorly by vein A1. In most Tephritidae, vein Cu2 is concave or has a distinct bend, forming an acute posteroapical extension on cell bcu. This cell was long commonly known as the ‘anal cell’ (e.g., Munro 1947) or the 1st anal cell, which is incorrect because it is anterior to vein A1. McAlpine (1981) called it the posterior cubital cell (cell cup) based on the untracheated structure (called vein CuP by McAlpine) that incompletely crosses the cell‘s anterior third. But at most, only the part posterior to ‘CuP’ should be called cell cup, but even that is questionable because the homology of the structure is uncertain (Steyskal 1984) and it does not reach vein Cu2 to form a complete cell. This cell has also been called the cubital cell or abbreviated as cell cu (e.g., Hardy 1973).
BASAL MEDIAL CELL (cell bm) – The basal wing cell bounded anteriorly by vein M, apically by crossvein bm-cu, and posteriorly by vein Cu. It has also been called cell M (e.g., Hardy 1973) or the 2nd basal cell.
BASAL MEDIAL-CUBITAL CROSSVEIN (crossvein bm-cu) – The more basal of the two transverse veins connecting veins M and Cu1, in Tephritidae located just distal to the fork in the cubital vein. This crossvein has also been called M3 or the anterior basal crossvein.
BASAL RADIAL CELL (cell br) – The elongate, basalmost radial cell, bordered anteriorly by the base of veins R and Rs and by R4+5, apically by R-M, and posteriorly by vein M. It has also been termed or abbreviated as cell R or the 1st basal cell.
CELL bcu – See basal cubital cell.
CELL bm – See basal medial cell.
CELL cu – See basal cubital cell.
CELL cup – See basal cubital cell.
CELL m – See medial cells and basal medial cell.
CELL sc – See subcostal cell.
COSTAL BAND (C-band) – A band along the anterior margin of the wing, typical of species presumed to be wasp mimics. It may vary in extent, e.g., it extends the entire wing length in Toxotrypana, from cell sc to the wing apex in most Bactrocera and Dacus spp., or from the wing base to the apex of vein R1 in most Anastrepha species. It is probably independently derived within various unrelated tephritid lineages.
COSTAL CELLS – A collective term for the basal costal cell (bc) and costal cell (c). 1st and 2nd costal cells (or 1st C and 2nd C) are old terms for these two cells, respectively.
CROSSVEIN dm-cu – See discal medial-cubital crossvein.
CROSSVEIN r-m – See radial-medial crossvein.
CROSSVEIN t-p – See discal medial-cubital crossvein.
CUBITAL CELLS – The cells posterior to the cubital vein; in Tephritidae the basal cubital cell (bcu) and the first cubital cell (cu1). The terms third posterior cell, anterior cubital cell, apical cubital cell and cua1 have been used for cu1.
CUBITAL VEIN/ CUBITUS (vein Cu) – According to McAlpine (1981), both the anterior and posterior branches of the cubitus are present in Diptera, but the homology of the untracheated structure he called the posterior cubitus (CuP) was questioned by Steyskal (1984), who considered it merely a sclerotized fold. The well developed part of the cubitus, technically CuA, has two branches, here abbreviated for simplicity as Cu1 and Cu2. Vein Cu1 has been termed or abbreviated as the cubitus, Cu, CuA1, fifth longitudinal vein, or M3+Cu. Vein Cu2 has been called the posterior basal crossvein, cu-an, or CuA2. In Tephritidae, it fuses with A1 to close the basal cubital cell. This combined vein has been termed the sixth longitudinal vein or Cu2+2ndA.
DISCAL CELL – See discal medial cell (dm).
DISCAL MEDIAL CELL (cell dm) – The cell near the middle of the wing bounded anteriorly by vein M, basally by crossvein bm-cu, posteriorly by vein Cu1, and apically by crossvein dm-cu. It has also been called cell 1st M2 (e.g., Hardy 1973), the discal cell, or the discoidal cell.
DISCAL MEDIAL-CUBITAL CROSSVEIN (crossvein dm-cu) – The more distal of the two transverse veins connecting veins M and Cu1, in Tephritidae usually located at about two-thirds of the wing length. It has also been called the hind posterior crossvein, lower crossvein, M crossvein, median crossvein, posterior crossvein, posterior transverse vein, vein im, vein m, or vein tp.
HYALINE – Clear, as in an unpatterned part of a wing. A hyaline wing means one with no markings.
MEDIA/ MEDIAL VEIN (vein M) – Technically the posterior medial vein, but in Diptera abbreviated for simplicity as vein M because the anterior branch of the media (MA) is very small. Although it is unbranched in most Cyclorrhapha, vein M may have up to three branches in other Diptera, and the single vein in Tephritidae has therefore sometimes been named M1 or M1+2. This vein has also been called the discoidal vein or 4th longitudinal vein.
MEDIAL CELLS – There are three cells posterior to vein M: the basal medial cell (bm), discal medial cell (dm), and medial cell (m). The latter has also been called the apical medial cell, cell am, 2nd M2, or the second posterior cell.
POSTERIOR CUBITAL CELL – See basal cubital cell.
PTEROSTIGMA – A sinus in the apical part of the subcostal cell that is often more opaque than surrounding areas. In Tephritidae it is the part of the cell distal to the bend of vein Sc. It has also been called the stigma or mediastinal cell.
RADIAL CELLS – In Tephritidae, there are four cells defined by the radial veins: the basal radial cell (br), and cells r1, r2+3 and r4+5. The latter two abbreviations are sometimes shortened to r3 and r5, respectively. Cell r1 has also been called the marginal cell or subcostal cell, cell r2+3 has been called the submarginal cell, and cell r4+5 has been called the 1st posterior cell.
RADIAL VEIN/ RADIUS – In Diptera, this vein may have up to five branches, but in Tephritidae only three are present. In Tephritidae it first divides into R1 (technically RA) and the radial sector (Rs), which divides into veins R2+3 and R4+5. The setation of the radial vein is taxonomically useful within the Tephritidae. R1 is finely setulose on the entire dorsal and sometimes part of the ventral side, and R2+3 and/or R4+5 may have setulae on either side. R1, R2+3 and R4+5 have also been called the 1st, 2nd and 3rd longitudinal veins, respectively, and when unbranced as in Tephritidae, R2+3 and R4+5 are sometimes shortened to R3 and R5.
RADIAL-MEDIAL CROSSVEIN (crossvein r-m) – The transverse vein connecting veins R4+5 and M. It has also been called the anterior crossvein, anterior transverse vein, median crossvein, or upper crossvein or abbreviated as i-m or ta.
S-BAND – A band in most Anastrepha species formed from a strongly oblique radial-medial band and the anterior apical band. It runs from cell bcu, diagonally across crossvein r-m to join the costa in the apical part of cell r1, and then follows the edge of the wing to the wing apex. It may be joined to the costal band and/or the V-band.
STIGMA – See pterostigma.
SUBCOSTA/ SUBCOSTAL VEIN (vein Sc) – The longitudinal vein between the costa and vein R1 on the anterior, basal part of the wing. A major diagnostic feature of the Tephritidae is that the subcosta is abruptly bent forward subapically and is weak beyond the bend. This vein has also been called the auxiliary vein or mediastinal vein.
SUBCOSTAL CELL (cell sc) – The cell bounded anteriorly by the subcosta and costa, and posteriorly and distally by vein R1. Also see pterostigma.
V-BAND – An inverted V-shaped wing band in most Anastrepha spp., formed from the subapical band (the ‘proximal arm’ of the V-band), which covers crossvein dm-cu, and the posterior apical band (the ‘distal arm’), which crosses cell m. They are usually joined in cell r4+5, but may be separated or the distal arm may be reduced or absent. In some species the V-band is joined to the S-band.
VEIN M – See media/medial vein.
VEIN R1 – See radial vein/radius.
VEIN R4+5 – See radial vein/radius.
VEIN Sc – See subcosta/subcostal vein.
WASP MIMICRY PATTERN – A wing pattern with a costal band and often an anal streak, which is presumed to mimic the appearance of vespid wasps or other stinging Hymenoptera.
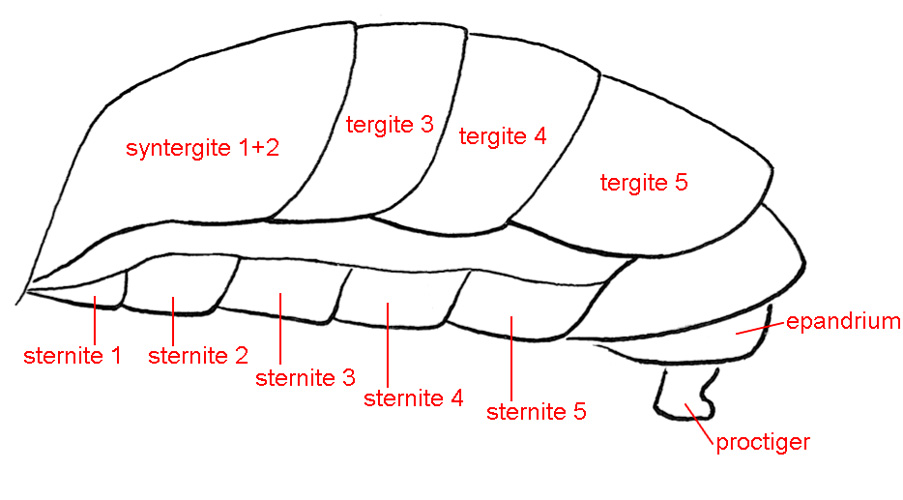
STERNITES – The ventral abdominal sclerites.
TERGITES – In the higher Diptera the first visible dorsal abdominal sclerite is formed by the fusion of tergites 1 and 2, and is called syntergite 1+2. In Tephritidae, the preabdomen of the male thus has four large tergites (1+2, 3, 4 and 5). Females have five, however tergite 6 may be short or be hidden under tergite 5 so that it is not visible from above.
TERMINALIA – See male terminalia, female terminalia.
SYNTERGITE 1+2 – See tergites.
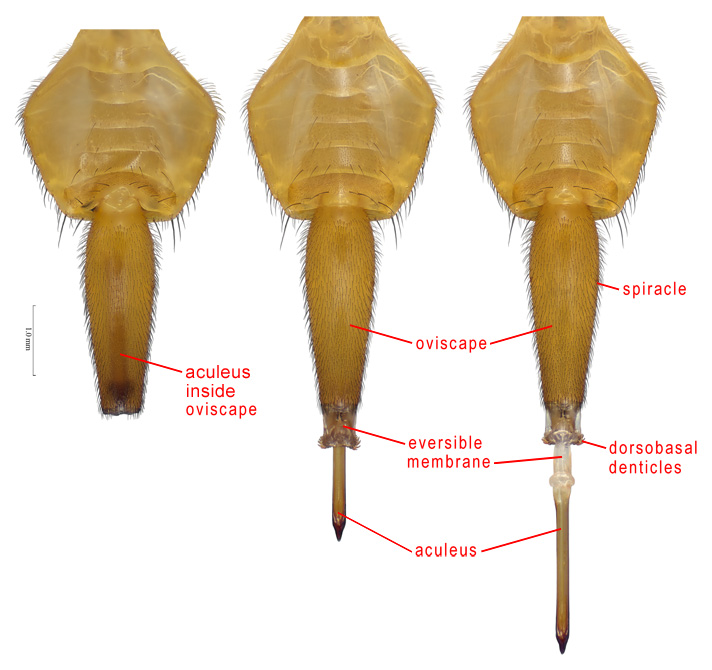
Female abdomen, ventral
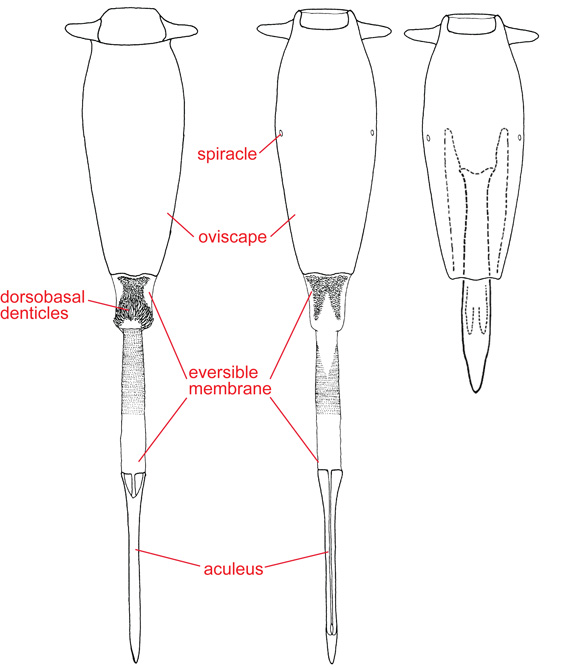
Female terminalia
ACULEUS – In Tephritoidea, the piercing part of the female ovipositor, which is normally retracted inside the oviscape (it often must be dissected to be examined). It consists of an elongate eighth tergite, a pair of elongate eighth sternites, which have also been called egg guides, genital flaps, valves, ventral flaps or ventral sclerites, and an apical cercal unit, a diamond-shaped or triangular part probably derived from the cerci and the subanal plate (hypoproct). In most Tephritidae the cercal unit is completely fused to the eighth tergite, but in most Phytalmiinae, it is free or there are sutures indicating the limits of these two sclerites. The aculeus has also been called the ovipositor, oviscapt, apical part of the ovipositor, piercer, ovipositor blade or gynium (see Norrbom & Kim 1988). Its ultrastructure and function were discussed by Stoffolano & Yin (1987).
ACULEUS TIP – The apical part of the aculeus. By convention, especially in Anastrepha, its length is measured on the ventral side from the inner margin of the sclerotized area, which has been erroneously called the apex of the oviduct (also see cloaca).
CLOACA/ CLOACAL OPENING – In most Diptera the genital and alimentary canals have separate openings. The genital opening is between segments 8 and 9 ventrally, and the anus is on the apical segment below the cerci (McAlpine 1981). In Tephritidae, the genital and alimentary canals join internally to form a cloaca, which opens on the aculeus between or just beyond the apices of the eighth sternites (Dean 1935, Stoffolano & Yin 1987, Valdez & Prado 1991). This opening in Tephritidae has been misnamed the genital opening (e.g., Norrbom & Kim 1988), which in these flies is at the base of the cloaca, or apex of oviduct (e.g., Stone 1942). Both eggs and waste are passed through the cloaca, and its opening is also the point of insertion for the male’s phallus during copulation.
EVERSIBLE MEMBRANE – The membranous part of the ovipositor between the oviscape and the aculeus. It is derived anteriorly from segment 7, and posteriorly from the intersegmental membrane between segments 7 and 8 (Foote & Steyskal 1987). It and the aculeus are normally retracted inside the oviscape, but they evert, at least partially, during oviposition and copulation. Basally the eversible membrane usually bears a dorsal and ventral pair of short sclerites, termed taeniae. The eversible membrane also bears minute, tooth-like scales or denticles, which may be simple, multidentate or comb-like. In Anastrepha and Toxotrypana, a group of dorsobasal denticles, varying in number and arrangement, are greatly enlarged. Stone (1942) used the term ‘rasper’ for this group of teeth, but their function for rasping is unproven and use of this term has been largely abandoned. Foote & Steyskal (1987) used the term more broadly for all of the denticles of the eversible membrane. The eversible membrane has also been called the eversible ovipositor sheath, inversion membrane, ovipositubus, or segment 8 (see Norrbom & Kim 1988).
EVERSIBLE OVIPOSITOR SHEATH – See eversible membrane.
FEMALE REPRODUCTIVE SYSTEM – The female reproductive system includes the external parts comprising the ovipositor, and internal parts, including paired ovaries and lateral oviducts, a common oviduct, 2–4 spermathecae and their ducts, accessory glands, and the genital chamber which bears the ventral receptacle and opens into the cloaca. See Dean (1935), Hanna (1938), Drew (1969), Dodson (1978), or De Carlo et al. (1994) for further details.
FEMALE TERMINALIA – See ovipositor.
GENITAL OPENING – In Tephritidae this occurs where the genital chamber opens into the cloaca. This term has often been misused for the cloacal opening in Tephritidae.
OVIPOSITOR – The parts of the female abdomen including and apical to segment 7, which are the main parts used in oviposition. In Tephritidae these are highly modified into three main parts: a tubular or conical oviscape; an elongate, membranous eversible membrane; and a needlelike or bladelike aculeus. The eversible membrane and aculeus are normally retracted, telescope-like, within the oviscape. Berube & Zacharuk (1983) and Stoffolano & Yin (1987) discussed the structure and operation of the ovipositor. The term ovipositor has been used by many American authors to mean only the aculeus. See Norrbom & Kim (1988) for discussion.
OVIPOSITOR SHEATH – See oviscape.
OVIPOSITUBUS – See eversible membrane.
OVISCAPE – The basal, tubular or conical segment of the ovipositor, which is formed by the fusion of tergite 7 and sternite 7. Technically it is syntergosternite 7 (Norrbom & Kim 1988), but the shorter term oviscape has gained wider usage. It bears spiracle 7 basolaterally and large internal phragmata basally. It has also been called the basal segment of the ovipositor or, especially by American workers, the ovipositor sheath.
OVISCAPT – A term which has been variously used in Tephritidae for the ovipositor, oviscape, or aculeus.
PIERCER – See aculeus.
RASPER – See eversible membrane.
SPERMATHECA (plural: SPERMATHECAE) – A usually sclerotized female internal organ used to store sperm. Tachiniscinae, Blepharoneurinae, Phytalmiinae and many Trypetinae (including Anastrepha and Toxotrypana) have three spermathecae, whereas Dacini, Tephritinae, and some Trypetinae have only two, and Oedicarena have four. They are normally found within abdominal segments 3–6, and are connected to the genital chamber by spermathecal ducts. The shape of the spermathecae varies considerably within Tephritidae, and their surface may be smooth, wrinkled, or covered by various papillose or dentate structures.
SPERMATHECAL DUCTS – Most of the Tephritoidea have two spermathecal ducts, of which the right apically bifurcates and connects to two spermathecae. In Pyrgotidae and Tephritidae there are two separate spermathecal ducts on the right side (3 total) that independently connect to the genital chamber. The apical portion of the spermathecal ducts is sometimes dilated, so the spermathecae may have the appearance of a figure 8 (e.g., Enicoptera, Celidodacus, some Trypetini).
SYNTERGOSTERNITE 7 – See oviscape.
TAENIA (plural: TAENIAE) – A paired, striplike, basal sclerite of the eversible membrane (Steyskal 1984).
VENTRAL RECEPTACLE – In Tephritidae, a small, sclerotized, multichambered structure on the vental side of the genital chamber. This has also been called the morula gland.
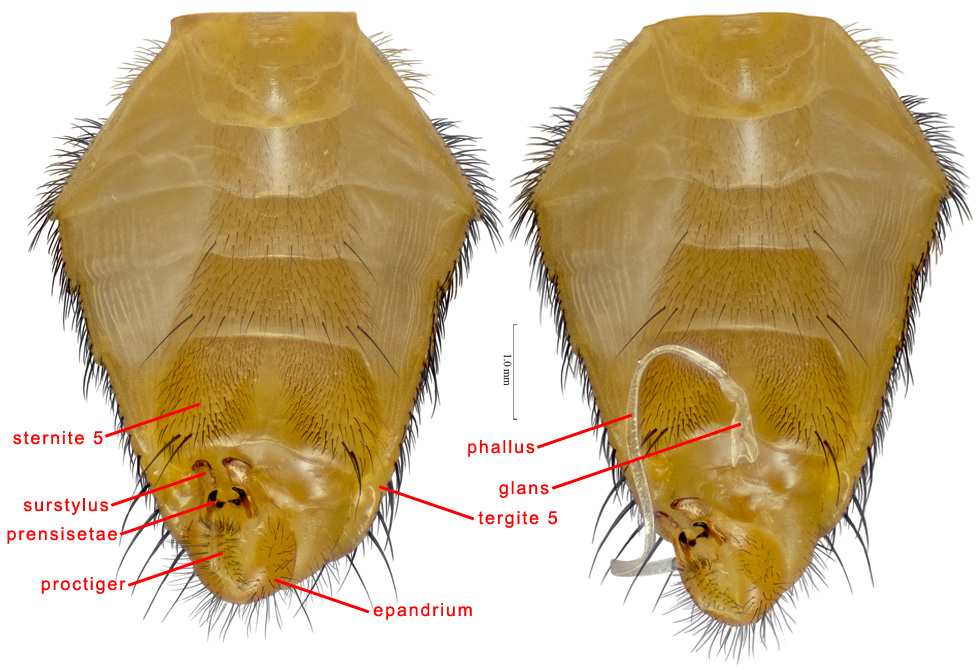
Male abdomen, ventral
Male abdomen, lateral
Male terminalia
AEDEAGAL APODEME – See phallapodeme.
AEDEAGUS – See phallus.
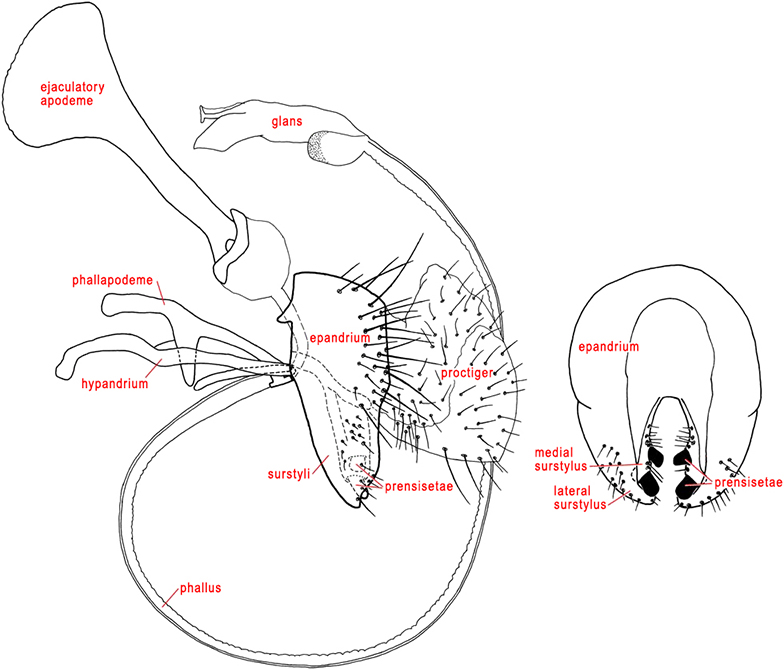
BASIPHALLUS – The very short, well-sclerotized, basal part of the male phallus. It has also been called the phallobase (Munro 1947). This term has also been used for all of the phallus except the glans (e.g., McAlpine 1981), but that homology is incorrect.
DISTIPHALLUS – The main part of the male phallus in Tephritidae. It includes an elongate basal part and an expanded, apical glans. The basal part, which coils at rest, has a pair of weak, elongate sclerites on the outer (posterior) side and numerous membranous folds on the inner (anterior) side. The term distiphallus has also been used to mean only the glans, but that homology is incorrect.
EPANDRIUM – The male abdominal tergite 9, which in Teprhitidae has a broad, inverted-U shape and bears paired surstyli ventrally or apicoventrally.
GLANS (plural: GLANDES) – The apical, expanded part of the male distiphallus. It usually contains complex internal sclerotization (acrophallus and praeputium) and may have a membranous vesica and a subapical lobe of various shapes. In many Trypetinae the glans has a membranous basal lobe (the preglans lobe of Korneyev 1996), usually covered with minute spicules, which in Ceratitis capitata expands and flexes during copulation, apparently to help insert the phallus by moving the glans through the female cloaca and genital chamber (Eberhard & Pereira 1998). The glans has also been called the aedeagal glans or the distiphallus, but it is only part of the latter.
HYPANDRIUM – The male abdominal sternite 9, which in Tephritidae is a slender, semicircular sclerite closely associated with the phallapodeme. It usually bears an anterior hypandrial apodeme and basolateral processes which have been termed lateral sclerites (possibly derivatives of the pregonites). The latter are not always fused to the hypandrium (e.g., in Anastrepha). The hypandrium has also been called the genital ring.
INNER SURSTYLUS – See medial surstylus.
LATERAL SCLERITE – A slender, paired sclerite of the male genitalia, possibly derived from the pregonite. It is usually basally fused to the hypandrium, but may be separate (e.g., in Anastrepha). Apically each lateral sclerite articulates with the apex of a lateral arm of the phallapodeme. Usually the right lateral sclerite is longer than the left.
LATERAL SURSTYLUS (plural: LATERAL SURSTYLI) – The more lateral of the two paired surstyli in the male genitalia (see surstylus). In Tephritidae the lateral surstylus is fused to the epandrium, sometimes to such an extent that the limits of these sclerites are unclear. In many Tephritidae the lateral surstylus has two lobes: an anterior lobe, which is recognizable by the presence of denticles and usually one or more sensilla; and a posterior lobe (Jenkins 1990). Occassionally it has a third, medial lobe. In Tephritinae it often has a posteriorly directed dorsal lobe basally, sometimes called the flange (Munro 1957).
MALE GENITALIA/ MALE TERMINALIA – In male Tephritidae, the genitalia are ventroapical and they may be hidden from above by the elongate tergite 5. The external parts include a broad epandrium, a slender hypandrium and associated lateral sclerites, an apical baglike proctiger, a paired lateral surstylus fused to the epandrium, a subepandrial sclerite joining a pair of medial surstyli that are closely associated with the lateral surstyli, an elongate phallus, and a phallapodeme with a pair of lateral arms. In Ulidiidae, some Platystomatidae and Pyrgotidae there are small, button-like, rudimentary gonostyli (= parameres of McAlpine 1981), each with 4–5 sensilla. In Tephritidae, they are usually completely absent, but are present in Tachinisca.
MALE REPRODUCTIVE SYSTEM – The male system includes the external male genitalia and the following internal parts: paired testes and vas deferens, several accessory glands, and an ejaculatory apodeme, sperm sac, and ejaculatory duct which connects to the phallus. See Hanna (1938), Drew (1969), Dodson (1978), or De Marzo et al. (1976) for further details.
MEDIAL SURSTYLUS (plural: MEDIAL SURSTYLI) – In the male genitalia, the slender lobe connected basally to the subepandrial sclerite and usually closely associated with the lateral surstylus. Sueyoshi (2006) indicated that it is homologous to the bacilliform sclerite of other Diptera. Subapically each medial surstylus bears a pair of prensisetae. It has also been called the inner surstylus.
OUTER SURSTYLUS – See lateral surstylus.
PHALLAPODEME – The sclerite which articulates with the base of the phallus (Cumming et al. 1995). In Tephritidae it bears, usually on its middle third, a pair of lateral arms or vanes that articulate apically with the lateral sclerites, which are usually fused to the base of the hypandrium. The lateral arms are sometimes fused basally to form a Y-shaped structure. The phallapodeme has also been called the aedeagal apodeme (McAlpine 1981) or fultella (Munro 1947).
PHALLUS – The male intromittent organ in Cyclorrhapha (Cumming et al. 1995), which in Tephritidae consists of a short basiphallus and a usually very elongate distiphallus. The length of the phallus is usually correlated with that of the female oviscape. At rest it is coiled and stored in a pocket above the postabdomen and below tergite 5. It has also been called the aedeagus (e.g., Foote & Steyskal 1987).
PRENSISETAE – Highly modified, short, stout setae on the medial surstylus. In Tephritidae there are usually two on each medial surstylus, usually located subapically.
PROCTIGER – In the male, the baglike structure attached posteriorly to the epandrium and subepandrial sclerite and bearing the anus. It is partly membranous, but ventrally and laterally it is weakly to moderately sclerotized, setulose and often microtrichose. It is sometimes involved in dispersion of pheromones produced by the rectum (see De Marzo et al. 1978). It has sometimes been called the cerci, and its sclerotized areas may be derived from them.
STERNITE 10 (male) – See subepandrial sclerite.
SUBEPANDRIAL SCLERITE – In Cyclorrhapha, a sclerite between the epandrium and the phallus (Cumming et al. 1995). In Tephritidae, this term has been used for the transverse sclerite connecting the basal ends of the medial surstyli, although Sueyoshi (2006) proposed that this structure is a composite of the dorsal bridge of the hypandrium and the subepandrial sclerite of other Cyclorrhaphan Diptera. The subepandrial sclerite has two anteriorly projecting lobes and one or two transverse connections or bridges. It has also been called sternite 10 (McAlpine 1981) or the interparameral sclerite (e.g., Norrbom & Kim 1988).
SURSTYLUS (plural: SURSTYLI) – In Cyclorrhapha, a paired clasping organ that articulates with the epandrium (Cumming et al. 1995). In Tephritidae it is divided into two parts that are usually very closely associated and difficult to distinguish. The lateral surstylus is fused to the epandrium, and the medial surstylus is fused to the subepandrial sclerite. During copulation they hold the female aculeus (see Eberhard & Pereira 1993, Headrick & Goeden 1995). The surstyli have also been called claspers (e.g., Stone 1942).
CHORION – The outer surface of the egg, which may appear smooth or reticulate.
MICROPYLE – A small, often nipple-like, structure at the anterior end of the egg where the spermatozoan enters.
PUPARIUM – The hardened skin of the last larval instar within which pupation takes place.
TENERAL – A freshly emerged adult with a soft, pale colored body and poorly formed wing markings, and often with the ptilinum exposed. Reared specimens should always be kept alive for a few days to allow their bodies to harden and colors to develop before being killed.
VITTA (plural: VITTAE) – The Latin term for stripe, which is a longitudinal color marking (as opposed to a band, which is transverse). Some authors have used the term vittae exclusively for the bright yellow or orange stripes on the scutum.
Berube, D. E. & R. Y. Zacharuk. 1983. The abdominal musculature associated with oviposition in two gall-forming tephritid fruit flies in the genus Urophora. Can. J. Zool. 61: 1805–1814.
Cumming, J.M., B.J. Sinclair & D.M. Wood. 1995. Homology and phylogenetic implications of male genitalia in Diptera – Eremoneura. Entomol. Scand. 26: 120–151.
Cumming, J. M. & D. M. Wood. 2009. Adult morphology and terminology, p. 9–50. In B. V. Brown, A. Borkent, J. M. Cumming, D. M. Wood, N. E. Woodley & M. A. Zumbado, eds., Manual of Central American Diptera, volume 1. NRC Research Press, Ottawa. p. xi + 1–715.
Dean, R. W. 1935. Anatomy and postpupal development of the female reproductive system in the apple maggot fly, Rhagoletis pomonella Walsh. N.Y. Agric. Exp. Stn. Tech. Bull. 229: 1–31.
De Carlo, J. M., G. N. Pellerano & L. I. Martinez. 1994. Saco del oviducto medio de Ceratitis capitata Wied. (Diptera, Tephritidae): consideraciones histo-funcionales. Physis (B. Aires) (1991) 49: 19–25.
De Marzo, L., G. Nuzzaci & M. Solinas. 1976. Aspetti anatomici, strutturali, ultrastrutturali e fisiologici delle ghiandole genitali accessorie del maschio di Dacus oleae Gmel. in relazione alla maturitą ed all’attivitą sessuale. Entomologica (Bari) 12: 213–240.
De Marzo, L., G. Nuzzaci & M. Solinas. 1978. Studio anatomico, istologico, ultrastrutturale e fisiologico del retto ed osservazione etologiche in relazione alla possibile produzione di feromoni sessuali nel maschio di Dacus oleae Gmel. Entomologica (Bari) 14: 203–266.
Dodson, G. 1978. Morphology of the reproductive system in Anastrepha suspensa (Loew) and notes on related species. Fla. Entomol. 61: 231–240.
Drew, R. A. I. 1969. Morphology of the reproductive system of Strumeta tryoni (Froggatt) (Diptera: Trypetidae) with a method of distinguishing sexually mature adult males. J. Aust. Entomol. Soc. 8: 21–32.
Eberhard, W. G. & F. Pereira. 1993. Functions of the male genitalic surstyli in the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). J. Kans. Entomol. Soc. 66: 427–433.
Eberhard, W. G. & F. Pereira. 1998. The process of intromission in the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). Psyche (1995) 102: 99–120.
Foote, R. H., F. L. Blanc & A. L. Norrbom. 1993. Handbook of the Fruit Flies (Diptera: Tephritidae) of America North of Mexico. Comstock Publishing Associates, Ithaca. 571 p.
Foote, R. H. & G. C. Steyskal. 1987. Tephritidae. In Manual of Nearctic Diptera, vol. 2 (J.F. McAlpine, ed.), pp. 817–831. Monograph of the Biosystematic Research Centre No. 28. Agriculture Canada, Ottawa.
Hardy, D. E. 1973. The fruit flies (Tephritidae – Diptera) of Thailand and bordering countries. Pac. Insects Monogr. 31: 353 p.
Headrick, D. H. & R. D. Goeden. 1995. Reproductive behavior of California fruit flies and the classification and evolution of Tephritidae (Diptera) mating systems. Stud. Dipterol. (1994) 1: 195–252.
Lima, A. M. da Costa. 1934. Moscas de frutas do genero Anastrepha Schiner, 1868 (Diptera: Trypetidae). Mem. Inst. Oswaldo Cruz Rio de J. 28: 487–575.
McAlpine, J. F. 1981. Morphology and terminology-adults. In Manual of Nearctic Diptera, vol. 1 (J. F. McAlpine, B.V. Peterson, G. E. Shewell, H. J. Teskey, J. R. Vockeroth & D. M. Wood, eds.), pp. 9–63. Monograph of the Biosystematics Research Institute No. 27. Agriculture Canada, Ottawa.
Munro, H. K. 1947. African Trypetidae (Diptera). A review of the transition genera between Tephritinae and Trypetinae, with a preliminary study of the male terminalia. Mem. Entomol. Soc. South. Afr. 1: [viii] + 284 p.
Munro, H. K. 1957. Sphenella and some allied genera (Trypetidae, Diptera). J. Entomol. Soc. South. Afr. 20: 14–57.
Norrbom, A.L. & K.C. Kim 1988. Revision of the schausi group of Anastrepha Schiner (Diptera: Tephritidae), with a discussion of the terminology of the female terminalia in the Tephritoidea. Ann. Entomol. Soc. Am. 81: 164–173.
Sabrosky, C. W. 1983. A synopsis of the world species of Desmometopa Loew (Diptera, Milichiidae). Contrib. Am. Entomol. Inst. 19 (8), 69 pp.
Steyskal, G. C. 1984. A synoptic revision of the genus Aciurina Curran, 1932 (Diptera, Tephritidae). Proc. Entomol. Soc. Wash. 86: 582–598.
Stoffolano, J. G., Jr. & L. R. S. Yin. 1987. Structure and function of the ovipositor and associated sensilla of the apple maggot, Rhagoletis pomonella (Walsh) (Diptera: Tephritidae). Int. J. Insect Morphol. Embryol. 16: 41–69.
Stone, A. 1942. The fruitflies of the genus Anastrepha. U.S. Dep. Agric. Misc. Publ. 439: 112 p.
Sueyoshi, M. 2006. Comparative morphology of the male terminalia of Tephritidae and other Cyclorrhapha. Isr. J. Entomol. 35–36: 477–496.
Valdez-Carrasco, J. & E. Prado-Beltran. 1991. Esqueleto y musculatura de la mosca del Mediterraneo, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Folia Entomol. Mex. (1990) No. 80: 59–225.
White, I. M. & M. M. Elson-Harris. 1992. Fruit Flies of Economic Significance; Their Identification and Bionomics. CAB INTERNATIONAL, Wallingford.
White, I. M., D. H. Headrick, A. L. Norrbom & L. E. Carroll. 1999. Glossary, p. 881–924. In M. Aluja & A. L. Norrbom, eds., Fruit flies (Tephritidae): Phylogeny and evolution of behavior. CRC Press, Boca Raton. [16] + 944 p.